Health
ParaGard IUD Removal Side Effects Lawsuit
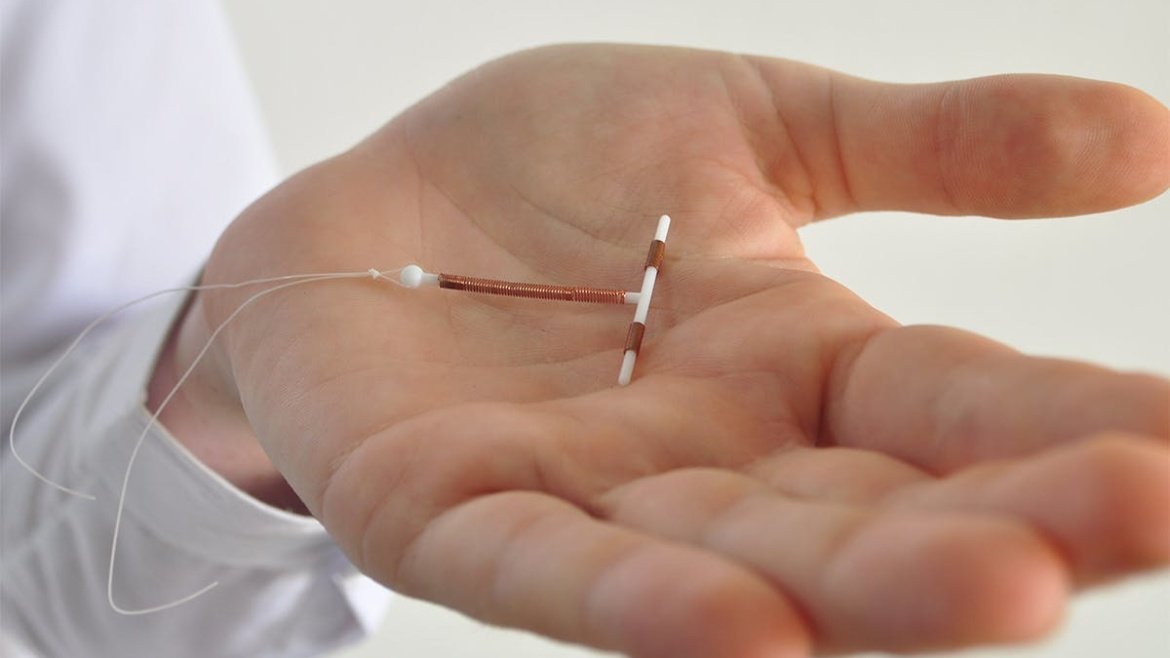
Paragard IUD is a copper-based, hormone-free, IUD that was approved for commercial use by the FDA in 1984 and has been recommended to thousands of women. However, several women have filed lawsuits against Paragard’s manufacturers for not providing adequate warnings about its risks.
Class action lawsuits
The manufacturer of the Paragard IUD has been accused of failing to warn women about the risks of removing the device. The lawsuits allege that Teva Pharmaceuticals knew of the potential risks and failed to warn their patients. The company is accused of not properly warning patients and should be held accountable for this.
A number of side effects have been reported, including the IUD sticking to the pelvic cavity, infections, and organ damage. In some cases, the IUD may migrate outside of the uterus, which can cause serious injuries, scarring, and even ectopic pregnancy. If this occurs, the woman can experience infertility and may even die from the complications.
Product liability lawsuits
Many women who have had side effects from Paragard are pursuing product liability lawsuits against the manufacturers. These lawsuits are based on the fact that the manufacturer didn’t adequately warn about the risks involved with the device. Consequently, a plaintiff may be entitled to compensation for pain and suffering, emotional distress, and birth defects. While there is no way to know exactly how much compensation you can expect for your injuries, a product liability attorney will be able to determine the likelihood of obtaining compensation for your injuries.
Typically, Paragard IUD lawsuits involve a discovery process that involves the introduction of evidence. Then, a pretrial conference is held to try to reach a settlement agreement, and a trial is held if a settlement cannot be reached. A consolidated group discovery process is overseen by the Multidistrict Litigation (MDL). This process aims to assess design flaws and whether the defendants knew of the complications.
Copper elements
The copper elements in ParaGard IUD removal side effects lawsuit has been filed against Teva Pharmaceuticals and CooperSurgical, Inc., the companies responsible for manufacturing the copper-infused device. The copper-infused device has been linked to severe emotional and financial problems in many women. The Showard Law Firm has experience representing women who have been harmed by defective medical devices.
Many women have experienced ongoing health problems and pain after their Paragard was removed. In some cases, the copper has migrated to other parts of the body.
Inflammation
If you are planning to remove your ParaGard IUD, it is important to know what to expect. Most women experience some pain, cramping, and plastic pieces in their bodies after the procedure. This problem has been highlighted in recent media reports. One such story features a woman who thought that the device was a low-maintenance birth control method.
This case study involved a 38-year-old Caucasian woman who underwent a paraGard copper IUD removal and endometrial biopsy. She had a previous history of cervical intraepithelioma, and positive HPV results. In addition, she had a family history of breast cancer and deep vein thrombosis.
Ectopic pregnancies
A ParaGard IUD removal lawsuit can be filed if you or a loved one suffered severe side effects from a failed procedure. One such lawsuit was filed by Georgia Bowers, who claimed that her IUD was not properly positioned inside her uterus, and broke during the removal process. Her lawsuit cited information in the package insert that described the risks of the procedure. A lawsuit is a way to demand justice for your condition and demand full compensation.
The Paragard IUD is a defective device. It can break into several pieces during the procedure and cause serious complications. The pieces can get stuck in your body and require surgery to remove. In addition, they can cause severe pain. In some cases, the fragments can get stuck in the uterus and require an immediate hysterectomy.
Organ damage
The Paragard IUD is a copper-coated intrauterine device (IUD) intended to prevent pregnancy. However, the device has a history of breaking and leaving metal and plastic debris inside the woman’s uterus after its removal. This has led to many women undergoing surgery to remove the metal fragment, but it has been found that the surgery was not always successful. If the metal fragment remains inside the woman’s body, it can pose serious health risks and even cause death.
The manufacturer of the ParaGard IUD device should have warned consumers of the potential risks associated with the device. The company could be held liable for the damage caused by the IUD.