This lawsuit against C.R. Bard et al. is scheduled to go to trial on February 21, 2023. The trial is expected to last three weeks, with each side allotted 35 hours of trial time. However, there is still a chance that the case could be settled before the trial begins.
C.R. Bard’s choice of polypropylene
In 1993, the company that produced heart catheters and polypropylene catheters was indicted on 391 counts of criminal activity. The company had sold defective catheters to patients without regulatory approval. Three of its executives were sentenced to prison on fraud and conspiracy charges, and the company paid $61 million in fines. The company has also been accused of manufacturing defective IVC filters and pelvic mesh.
Bard’s choice of polypropyethylene for its surgical mesh products comes from its subsidiary Davol. Davol was founded in Providence, Rhode Island, in the 1870s as Perkins Manufacturing Company, which later changed its name to Davol Rubber Company. Originally a manufacturer of surgical devices and catheters made of rubber, Davol soon became one of the largest brands in this market.
The company is named in several polypropylene hernia mesh lawsuits. Many of these lawsuits have been consolidated into multidistrict litigation (MDLs) in an effort to streamline litigation and reduce costs. However, each plaintiff retains the right to litigate their claims in court if necessary.
Adhesions
The Bard Mesh litigation owes its existence to the Kugel Mesh litigation. In the first bellwether trial, Bard won a surprise victory, but the plaintiff prevailed in the second trial and received a $1,500,000 verdict. That victory forced Bard to agree to a global settlement of all cases.
The plaintiffs claim that the company failed to properly test its products. They allegedly used unsuitable plastic that was not suitable for use in permanent implantable products. The company’s plastic supplier warned it not to use this type of plastic, but Bard executives insisted on its use.
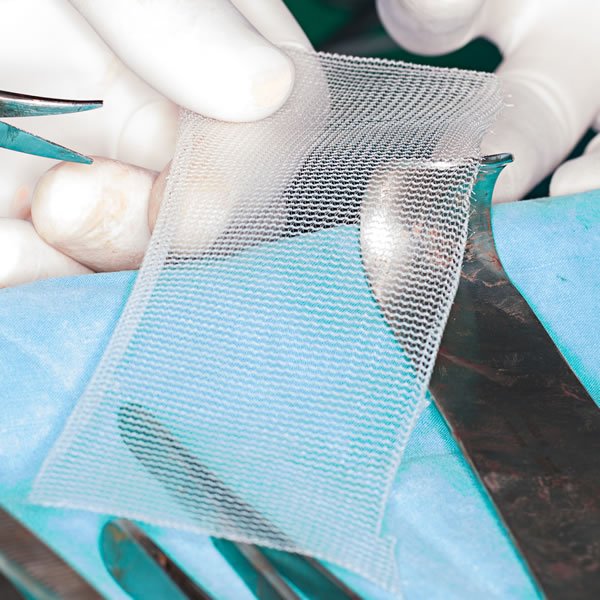
The Bard Ventralex hernia mesh implant consists of a self-expanding patch with two layers of polypropylene mesh stitched to a polytetrafluorethylene sheet. Because of the impermeability of the plastic, fluid cannot escape, leading to infection and seroma formation.
Fistulas caused by Bard Mesh
Fistulas are an abnormal connection between two body parts, which can be either internal or external. They can be present from birth or can develop as a result of surgery, injury, infection, or certain diseases. They can also result from defective surgical mesh or hernia mesh. In some cases, the fistulas can cause no symptoms, while others may require corrective surgery.
The initial treatment is the drainage of the abscess, followed by antibiotic therapy. In this case, the mesh penetrated the small intestine. The patient was discharged 24 days after the surgery. Histological examination showed that the mesh had penetrated the small intestine and colon, causing the small intestine and colon to lump together.
Fistulas caused by Bard mesh are uncommon, but can occur. These complications are caused by improper fixation of the mesh in previous hernia surgery. In addition, the mesh may have become deformed due to the gap between the abdominal wall and the mesh, or because the mesh was distorted during suturing. To minimize the risk of fistula formation, peritoneal coverage is essential.